As the EU Interreg project ESS & MAX IV: Cross-Border Science & Society comes to an end, we highlight the work of another young scientist whose research has benefitted from the project’s flagship programme MAX4ESSFUN.
Last Friday marked the closing of the highly successful European Union Interreg project ESS & MAX IV: Cross-Border Science and Society. The project’s scientific research component, MAX4ESSFUN, supported 194 trans-national research projects in the Öresund-Kattegat-Skagerrak (ÖKS) region, which includes parts of Sweden, Denmark and Norway.
In cooperation with the European Spallation Source (ESS) and the MAX IV synchrotron in Lund, the Cross-Border Science and Society project worked for three years to build user capacity for the two facilities. It has now established a long-term platform for this cross-border research to continue.
“The biggest outcome of the project for us is the awareness it has created of the facilities, and why it is necessary to make them a success,” said ESS Director General John Womersley in his address at the project’s August 21 closing conference. “Also, the training of the potential user community which the Interreg project has initiated is of extreme importance to the facilities.”
The €19 million project directly financed scientific experiments, training and supervision for PhD and postdoc researchers at eight universities in the ÖKS region. Led by Sweden’s Region Skåne and co-lead partner Region Hovedstaden, in Denmark, the project included 27 partner organisations in the region, including the participation of several scientists at ESS.
Protein characterisation to combat skin diseases
Kristian Stødkilde-Jørgensen is a postdoc in Århus University’s Department of Biomedicine. His chief interest is in research that focuses on medical patients and disease, with the goal to “carry out basic research that is commercially interesting and that everyone in society can benefit from”.
With contributing funds from MAX4ESSFUN, Stødkilde-Jørgensen has spent two years investigating a newly discovered protein that may have implications for treating a variety of skin diseases, including psoriasis.
The ESS instrument NMX, a macromolecular diffractometer, will use the high neutron brightness of ESS to open up new possibilities for structural biology research with neutrons. Additionally, ESS is working with Lund University and other partners to improve protein crystallisation and deuteration techniques. This is an important initiative at ESS that will open up studies like Stødkilde-Jørgensen’s to the benefits of neutron scattering.
This interview was first published in Danish by ESS & MAX IV: Cross Border Science and Society on March 2, 2018, and has been lightly edited.
Young scientists in MAX4ESSFUN: Kristian Stødkilde-Jørgensen
March 2, 2018
ESS & MAX IV: Cross Border Science and Society
Young researcher: Kristian Stødkilde-Jørgensen, Århus University
Supervisor: Christian Brix Folsted Andersen, Århus University
Co-supervisor: Mattias Colin, Lund University
Kristian Stødkilde-Jørgensen works as an assistant professor at the biomedical institute at Århus University (AU), where he also studied molecular biology, specialising in the field of biochemistry. Kristian has always been interested in research that focuses on patients and diseases, which is why he calls himself a "closet physician".
Kristian was contacted by Holger Brüggemann, from the Biomedical Institute at AU, and Rolf Lood, from Lund University, as they had discovered a new and unique protein and wanted Kristian's help to investigate it further. The unique aspect of this protein is that its sequence structure is unlike that of any other protein, so there are also no hintsin the available literature that can explain how the protein works.
"So if and when we discover how the protein works, it will of course provide us with some completely new basic knowledge on this type of protein,” explains Kristian. Funds from MAX4ESSFUN and from the Novo Nordisk Foundation have made it possible for Kristian to research the protein properties and structure for two years.
The uniqueness of the protein
Through his research, Kristian is trying to understand the interaction between a particular bacterium (which produces the protein), which all human beings have on their skin, and the general health of the skin. The body does not kill the bacterium, which has led to speculation as to whether the bacterium can have a beneficial effect on the skin.
A possible beneficial effect concerns the sun's ultraviolet rays. When UV light comes into contact with the skin, some potentially dangerous reactive molecules are created. The bacterial protein may arguably help neutralise these reactive molecules and thereby help keep the skin healthy.
Kristian says that an absence of the bacterium has also been associated with the development of, among other things, psoriasis, and he therefore wonders if the lack of protein may contribute to the development of some types of skin diseases.
Kristian’s dream
Kristian is an entrepreneur at heart, and is playing with the idea of starting a business where some of the new knowledge acquired through his research can be used to develop new treatment possibilities.
“If we find out there is a correlation between the protein that the bacterium produces and some types of skin diseases, it would be obvious to investigate whether we can steer this knowledge from the laboratory to a real product that can benefit people.”
If the entrepreneurial dream does not become reality, Kristian aims to remain at university and start a research group, with a special focus on research having a product-oriented approach.
“I'm motivated by the fact that what you tinker with can be used in society for the benefit of many.”
Technology and research
Experiments with the protein have been carried out by Kristian and his research team at the DESY synchrotron in Hamburg and ESRF in Grenoble, and Kristian looks forward to continuing his research at MAX IV.
The X-ray at MAX IV is so powerful that there is the potential for better data than at other synchrotrons around Europe. The X-ray’s strength will be of significant benefit in Kristian's experiments, as the protein crystals are very small and difficult to optimise, which has made it difficult to obtain data that can reveal the structure of the protein.
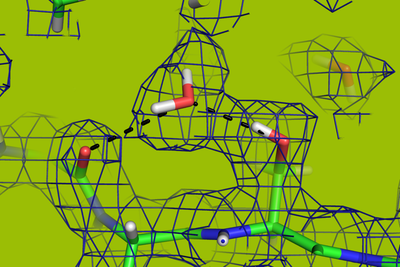
Specifically, Kristian goes in and converts the protein from the bacterium to a protein crystal.Then he fires the crystal with X-rays to find out where the electrons of the protein are, and subsequently how the individual atoms are placed in relation to each other.
When Kristian has this data, he can map the three-dimensional structures of the protein and document the function of the protein all the way down to an atomic level. The way in which the building blocks of the protein are folded together in the three-dimensional structure is that which gives the protein its unique properties.
The collaboration opens up opportunities
Kristian has benefited greatly from the collaboration across the Öresund, which MAX4ESSFUN has made possible. The collaboration results in research at a higher level, as researchers with different areas of expertise can be involved, thereby allowing a broader perspective and better sparring, he says.
At Århus University, specialists in deriving a protein's spatial structure can be found. At Lund University, where Kristian’s co-supervisor Mattias Colin is an associate professor in the Division of Infection Medicine, they have specialised knowledge on bacteria, the immune system and how proteins work.
Kristian has also collaborated with Oslo University through the MAX4ESSFUN programme.
“If it had not been for the MAX4ESSFUN funds, we would probably not have had the opportunity to involve those from Oslo in the project. But they have some really useful techniques up there, which we absolutely do not have the opportunity to have here in Århus.”
Kristian explains that knowledge on what they are able to do at the various universities has meant that those across the Sound have also begun to collaborate on other projects relating to proteins. The network created through MAX4ESSFUN has resulted in Kristian assisting in projects he would not otherwise have been asked about.
Text: Axelina Kull, Region Skåne
Translation: Marie Powell, ESS