The EU's RAMP project interviews PhD student Swati Aggarwal, who is working at ESS to optimise crystallisation methods for proteins key to understanding the basic biological processes of proton pumps. Supported by the EU program, Aggarwal’s research will help to open up the potential of the groundbreaking ESS instrument NMX.
- Membrane proteins form more than 85% of drug targets, but just 600 unique membrane protein X-ray crystal structures have been determined. A better understanding of how to crystallize membrane proteins reliably is therefore urgently required.
- Swati Aggarwal is a PhD student working at ESS supported by the RAMP (RAtionalising Membrane Protein crystallization) Innovative Training Network funded by the Marie Skłodowska-Curie Actions of the EU's H2020 programme.
- Aggarwal is supervised by ESS instrument scientist Esko Oksanen, originator and lead scientist for the instrument NMX, a macromolecular diffractometer under construction at ESS. Designed to identify the position of hydrogen atoms in molecules never probed with neutrons before, NMX will be a game-changer in structural biology.
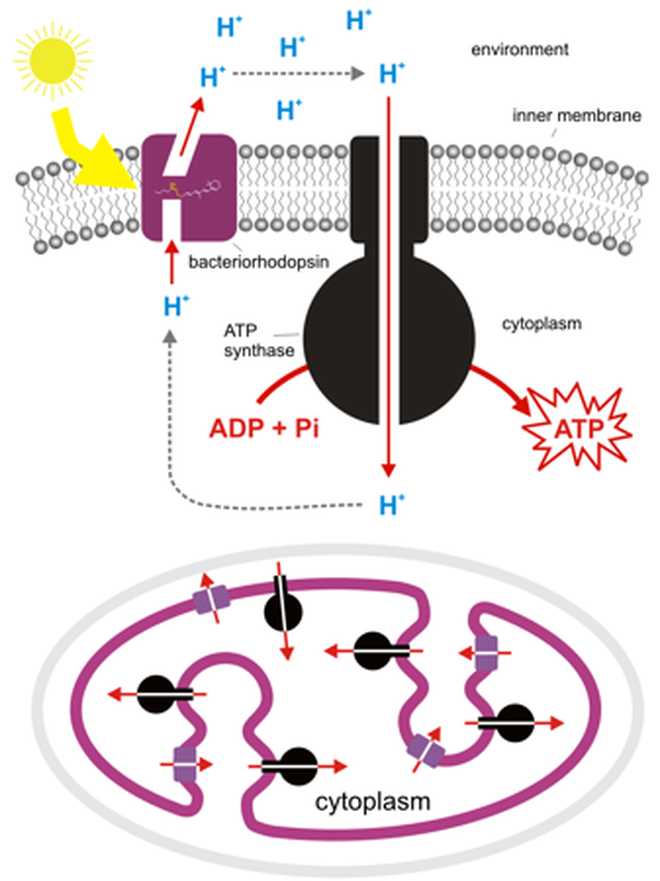
- One key to maximising the potential of NMX is to optimise crystallisation techniques that are used to prepare a protein for neutron studies. Aggarwal is working on such techniques with two proteins that function as proton pumps—the catalysts for energy production in cells.
- Proton pumping is a biological system in need of deeper understanding, both for the advancement of fundamental cell biology and also in the development of critical drug therapies.
This interview is reprinted courtesy of the RAMP Network and was originally published on the project website.
Interview: Swati Aggerwal
RAMP Innovative Training Network
JAN 17, 2018
Early Stage Researcher (ESR) 11: Swati Aggarwal
Institution: European Spallation Source (ESS) ERIC in Lund, Sweden
PhD subject: Elucidating the function of proton pumps with neutron crystallography
Please tell us about yourself.
I come from a small valley surrounded by the Himalayas known as Dehradun in India. I completed my Master’s in Biotechnology from National Institute of Technology, Rourkela, India. I spend my leisure time in painting and cooking—the spicy Indian cuisine. I aspire to travel and explore the world as much as I can.
Why are you interested in science?
Science is the answer to every question that we come across since our childhood to adulthood. Being a child, we think, “Why the lizard can grow its tail again and not us if we lose any part of our body?” “Why the sky is blue?” “Why can’t we keep a football in the air?” As we grow, science solves all our queries. It is the way of understanding the world around us. The more we understand, the more questions arise to go into depth. Our urge to look for answers is a never-ending process, especially for the little girl inside me who is always in the state of asking “Why.” So, this is what motivates me to be in science.
Please tell us about your PhD project.
Proteins are responsible for about 15% of the average mass of a human body. They are the building blocks of the body and major constituents of muscles, ligaments and hair. Classification of proteins is based on their function, structure, shape and solubility. We are interested in membrane proteins that reside on the cell membrane and are classified on the basis of their solubility. These proteins perform vital functions like acting as transporters and receptors of molecules, transducing signals and energy across the membrane.
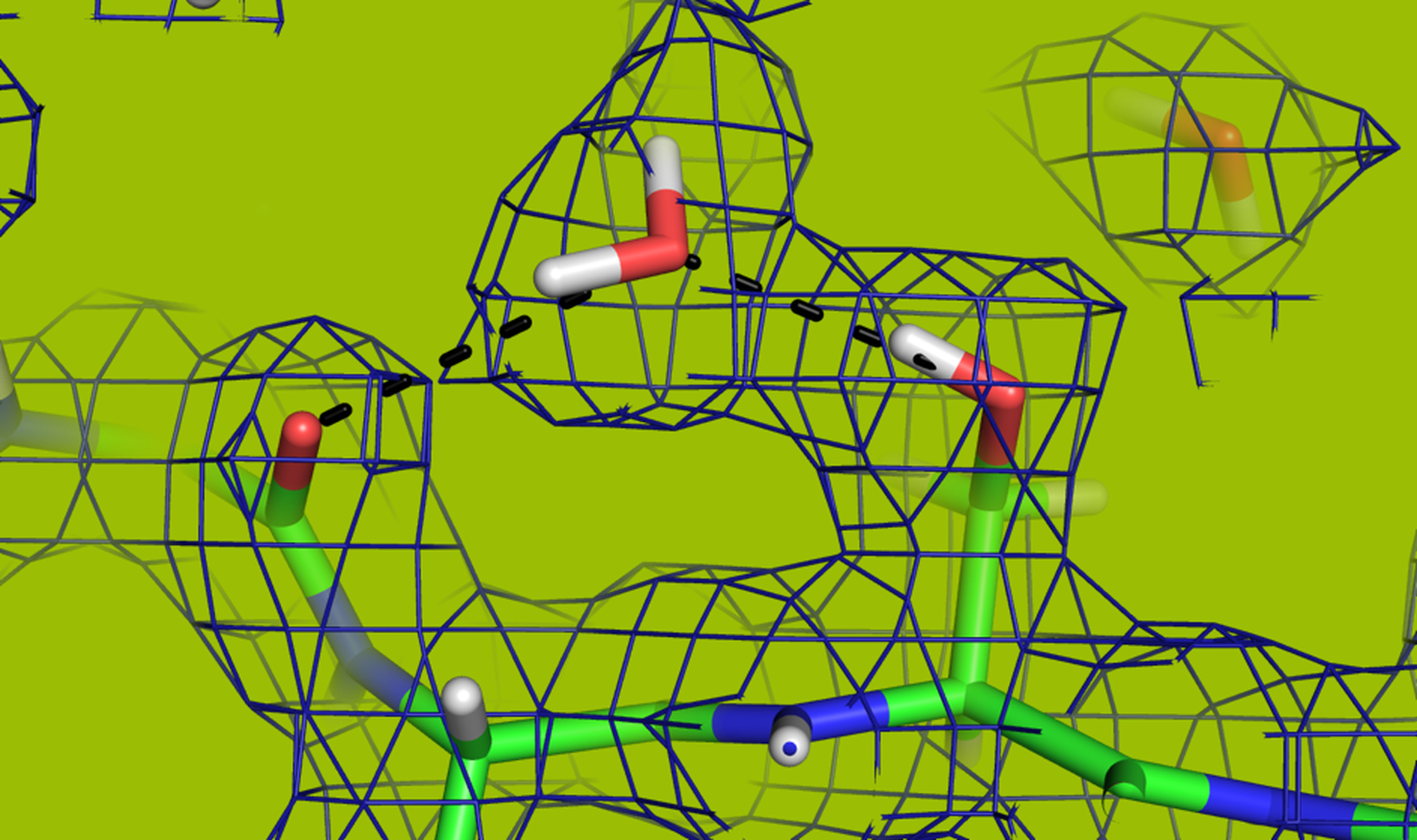
Energy is essential for our body and a few membrane proteins act as proton pumps and help to generate chemical energy by building a potential gradient across the membrane. Hydrogen plays an important role in the function of proton pumps such as bacteriorhodopsin (BR) and cytochrome C (COX). Thus, it becomes really important to study the hydrogen positions by determining their structure. X-Ray Crystallography is the most commonly used technique to study the structure of proteins, though hydrogen positions are not visible by X-Ray, so, neutron diffraction solves the problem.
But the challenging part is the requirement of larger and well-defined crystals formed with a robust process. Necessary methods to improve crystal size and diffraction quality will be developed in order to have a deeper understanding of BR and COX.
What do you enjoy most in your position within the RAMP Network? Why?
As the name suggests, ITN, innovative training network, is the best example of brain-storming, where people with distinct expertise join at the same platform for a common cause. Most importantly, we get a chance to interact and know people from different cultures across the world. Being in this network, we not only gain technical knowledge while working on our projects, but also develop professional skills to make a difference in industry or academics in the near future.
THIS PROJECT HAS RECEIVED FUNDING FROM THE EUROPEAN UNION'S HORIZON 2020 RESEARCH AND INNOVATION PROGRAMME UNDER THE MARIE SKŁODOWSKA-CURIE GRANT AGREEMENT NO 722687