NMX
Macromolecular Diffractometer
The NMX macromolecular diffractometer will open new avenues for structural biology with neutrons. Determining hydrogen atom positions faster than before from smaller crystals and larger unit cells than before will allow studies of more challenging systems such as transmembrane proton pumps. This will yield an improved understanding of not only fundamental biological processes such as energy production in cells, but also the way in which pharmaceuticals bind their target proteins.
Instrument Class
Beam Port
Lead Scientist
Lead Engineer
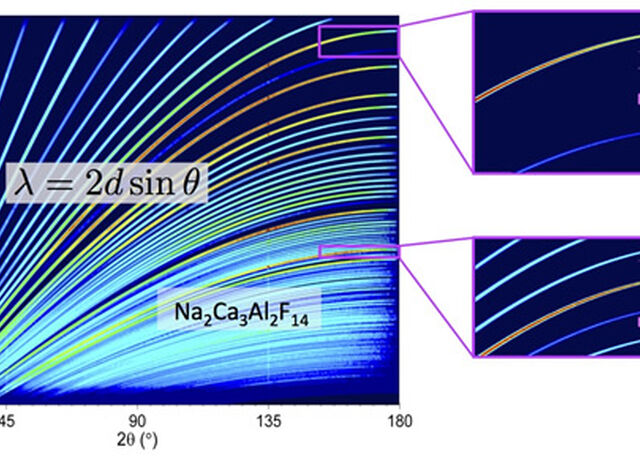
The NMX Macromolecular Diffractometer is a time-of-flight (TOF) quasi-Laue diffractometer optimised for small samples and large unit cells dedicated to the structure determination of biological macromolecules by crystallography. The main scientific driver is to locate the hydrogen atoms relevant for the function of the macromolecule.
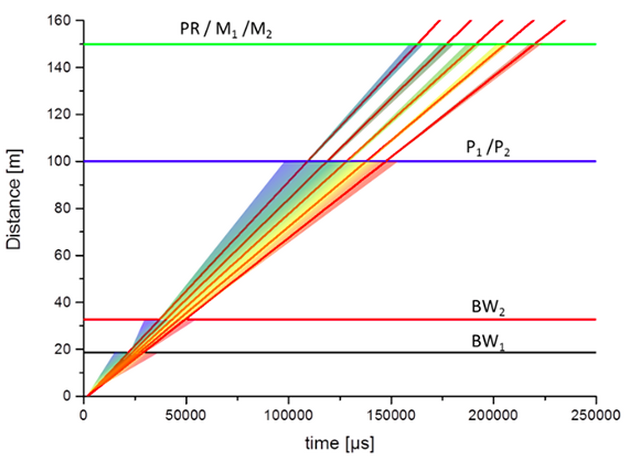
The ESS long pulse source is well suited for a quasi-Laue macromolecular diffractometer that can spread the background in the TOF dimension, while the Bragg peaks are observed at a defined TOF. Therefore a macromolecular diffractometer at the ESS could be used either to study systems with smaller crystals or larger unit cell volumes. Growing well-ordered protein crystals of cubic millimeter volume is extremely difficult, so the instrument is optimised for submillimeter crystal sizes.
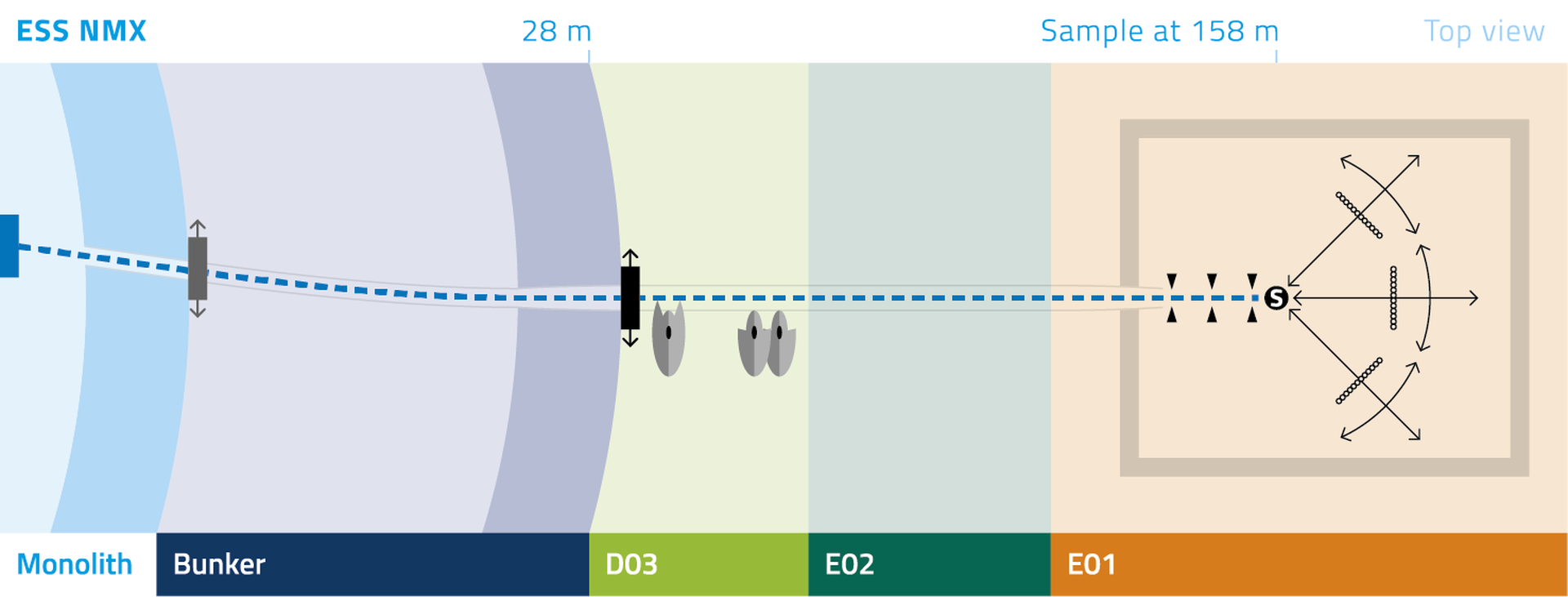
One of the limiting factors with current instruments is that the fixed detector geometry only allows a maximal unit cell edge of ~150 Å to be resolved without a compromise in the diffraction resolution (dmin). The NMX instrument allows larger unit cells to be resolved by increasing the crystal-to-detector distance. This incurs an increase in the data collection time, but reflections to the same dmin can still be observed by swinging the detector in scattering angle 2q.
Many of the scientifically most interesting systems, such as proton pumping membrane proteins, crystallise in large unit cells, so simply being able to resolve a large unit cell edge is a unique advantage. The combination of a neutron flux comparable to leading high flux reactor instruments, such as LADI-III, together with time-of-flight expansion, the ability to resolve large unit cells and the ability to separate signal from background leads to world-leading performance particularly with the experimentally most challenging systems.
The ESS NMX instrument will transform neutron macromolecular crystallography into a technique that answers a significantly larger number of hydrogen-related questions in biomolecular science than before.
The NMX Macromolecular Diffractometer is a single crystal diffractometer using the quasi-Laue time-of-flight (TOF) technique.
The main parts of the instrument are:
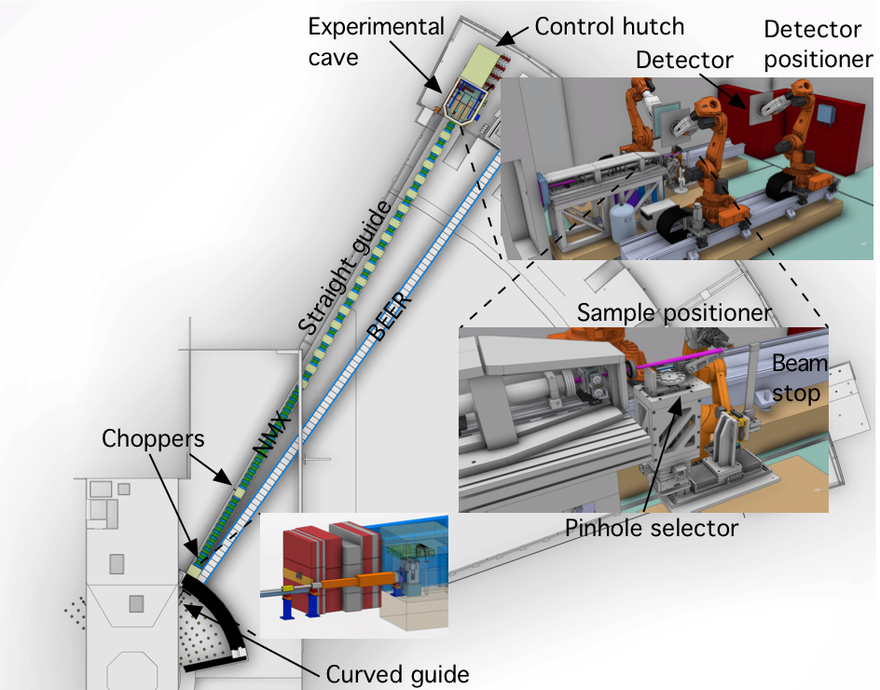
- the beam transport and conditioning system (BTS) that delivers the neutron beam to the sample position in the experimental cave
- the sample exposure system (SES) that positions the sample crystal in the neutron beam
- the scattering characterisation system (SCS) that detects the diffracted neutrons
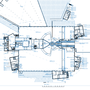
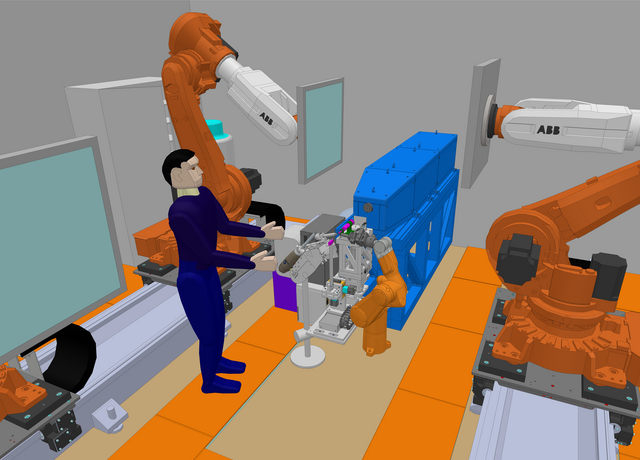
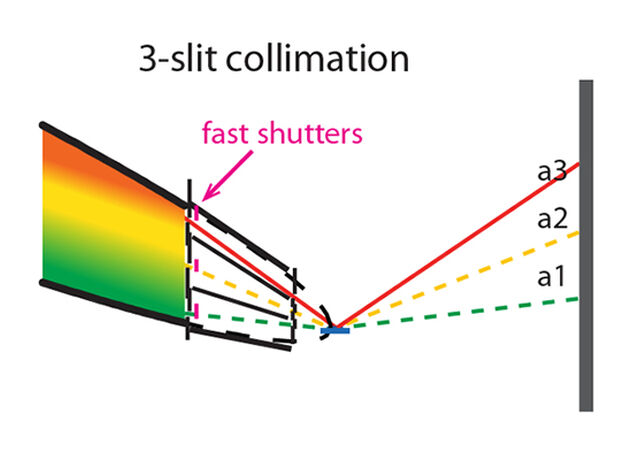
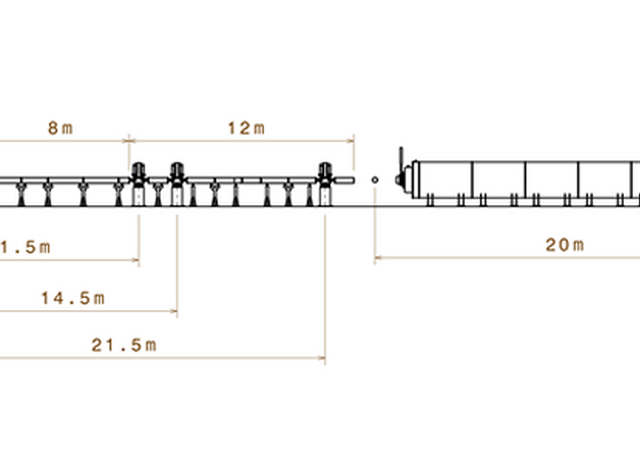
The BTS consists of a neutron guide system where a curved part (Figure 1) loses the line-of-sight to the neutron moderator inside a common shielding bunker and a straight part delivers the beam to the experimental cave. A chopper system selects the desired neutron wavelengths. Inside the experimental cave a collimation system tailors the divergence and size of the neutron beam to match the sample properties—the beam size can be selected with a pinhole exchanger (Figure 1). The sample is positioned and rotated with a robotic sample positioner (Figure 1) and the three 50 x 50 cm detector panels are also mounted on six-axis robots. The experiment will be controlled and samples prepared in an adjacent control hutch.
Sample Environment
Data collection will be possible at ambient as well as cryogenic temperatures and a humidity control device is foreseen.
Publications
Andersen, K. H. et al. The instrument suite of the European Spallation Source. Nuclear Instruments & Methods in Physics Research Section a-Accelerators Spectrometers Detectors and Associated Equipment 957, 39, doi:10.1016/j.nima.2020.163402 (2020).
Lead Scientist
Esko Oksanen, ESS
Lead Engineer
Guiseppe Aprigliano, ESS
Scientists
Jean-Luc Ferrer, Institut de Biologie Structurale, Commissariat à l’énergie atomique et aux énergies alternatives (CEA)
Peter Zagyvai, Energy Research Centre of the Hungarian Academy of Sciences
Szabina Török, Energy Research Centre of the Hungarian Academy of Sciences
Gábor Nafradi, Energy Research Centre of the Hungarian Academy of Sciences
Márton Markó, Wigner Research Center for Physics of the Hungarian Academy of Sciences
Petri Kursula, University of Bergen
Physics Engineer
Dorothea Pfeiffer, ESS
Neutron Beam & Shielding Scientist
Valentina Santoro
Engineers
Markus Olsson, ESS
Endre Kosa, Wigner Research Center for Physics of the Hungarian Academy of Sciences