ESS researchers will apply the high brilliance of the ESS source to understand life processes in greater detail than ever before.
- This is the ninth in a series of articles highlighting the instruments selected for the European Spallation Source suite of instruments. Twenty-two instruments are anticipated at ESS, chosen to address the needs of the ESS core user community. They have been selected through a rigorous but inclusive, community-based process that begins with an open call for proposals, moves through reviews by experts inside and outside ESS, and ends with a recommendation to advance into Phase 1 by the ESS Council.
Life is sustained by enzymes. Mapping out the structure of these large, complex molecules lets us understand how they work as well as how they malfunction, increasing our understanding of life processes and helping us fight disease.
Macromolecular crystallography, the structural study of large biological molecules, is a workhorse discipline at synchrotron X-ray sources globally. As a next step of scientific discovery, researchers will regularly utilise high flux neutrons to accurately locate the hydrogen atoms in these biomolecules, and therefore improve our understanding of complex biological mechanisms. NMX, at the European Spallation Source (ESS), will be the instrument to singularly elucidate these details of life.
“Hydrogens are extremely important in biology. There are hydrogen bonds everywhere, where individual atoms or two molecules interact, and inside the folded structure of a biomolecule tertiary interactions are based on H-bonds. There’s [also] water everywhere, biological structures and reactions only really take place due to interactions with water,” says Poul Nissen, a protein biochemistry professor at Aarhus University in Denmark, who assisted in the visionary phase of NMX. Nissen’s studies focus on active transport systems such as calcium pumps and sodium-potassium pumps that are membrane proteins.
Essentially named for neutron macromolecular crystallography, the NMX instrument is particularly valuable for life science research. Its construction timeline corresponds to the highly anticipated start of the ESS User Program in 2023. The project team, led by ESS instrument scientist Esko Oksanen, has been one of the forerunners of an adaptive, ongoing design process orchestrated for the entire instrument suite of ESS. The project is currently nearing completion of the detailed engineering design phase. Next steps will involve In-Kind procurements through contracting with ESS partners.
“ESS has many In-Kind partners working to make NMX a success,” says Oksanen. “For instance, we have nice progress on the robotics from the Institute for Structural Biology (IBS) through the French Alternative Energies and Atomic Energy Commission (CEA) in Grenoble. We have our Hungarian partners at the Wigner Research Centre for Physics developing the beam delivery system optics and choppers. The Centre for Energy Research in Budapest is doing the shielding calculations and design.”
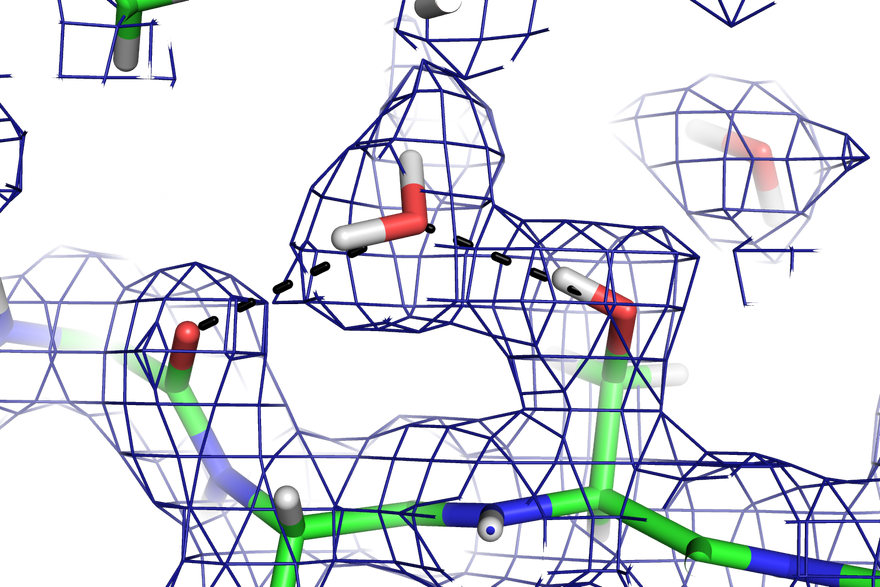
NMX is a single crystal diffractometer that measures the structure of large biological molecules, such as proteins, with the primary goal to spatially locate the hydrogen atoms. Attaining this knowledge is considered the key to unlocking fundamental cellular processes such as enzyme catalysis and protein-ligand interactions.
The instrument provides atomic resolution information from sub-millimetre crystals with large unit cell dimensions greater than 150 Ångstroms – which is currently a measurement limitation for most membrane protein crystals structures, such as proton pumping enzymes. It is possible by increasing the distance between the sample and neutron detectors through the use of versatile robotic arms and by time-of-flight data collection modes. This instrumental flexibility even ensures that magnetic materials research, using similarly small single crystals and requiring large magnetic units cells to be studied, is also well served with NMX.
Benefits of the long pulse
“It was not immediately obvious that the ESS long pulse source design could accommodate NMX. The recommendation of the 2002 ESS Technical Advisory Committee was that NMX should be on [a] short pulse 5 Mega Watts source,” says John Helliwell, emeritus professor at the University of Manchester in the UK and chair of the ESS Scientific and Technical Advisory Panel (STAP) for macromolecular crystallography. “The core issue was the resolvability of Laue diffraction spots stimulated by a long time pulse for the large unit cell sizes and diffraction resolution limits to be measured in structural biology.”
Prior to an ESS STAP review of the NMX instrument proposal in 2013, these parameters were carefully examined using Daresbury Lauegen software developed for synchrotron radiation x-ray Laue crystallography by Helliwell’s team at the Science and Technology Facilities Council (STFC) Daresbury Laboratory in the UK. “The simulations categorically showed not only the feasibility of NMX on the ESS long pulse but also the big advantage of the high intensity of neutrons in a long pulse for the NMX experiments,” explains Helliwell.
Macromolecular crystallography studies with neutrons have classically been limited by a lack of flux, availability of large sample sizes, and the signal-to-noise ratio for the sample that is possible to achieve. The NMX instrument operates with the time-of-flight (ToF) technique–a measure of time that neutrons travel from source to detectors—which reduces background for data collection. The ToF technique also allows improved study of crystals unable to undergo perdeuteration, a standard process where the hydrogen in a molecule is replaced by deuterium for lower background.
Optimised data collection
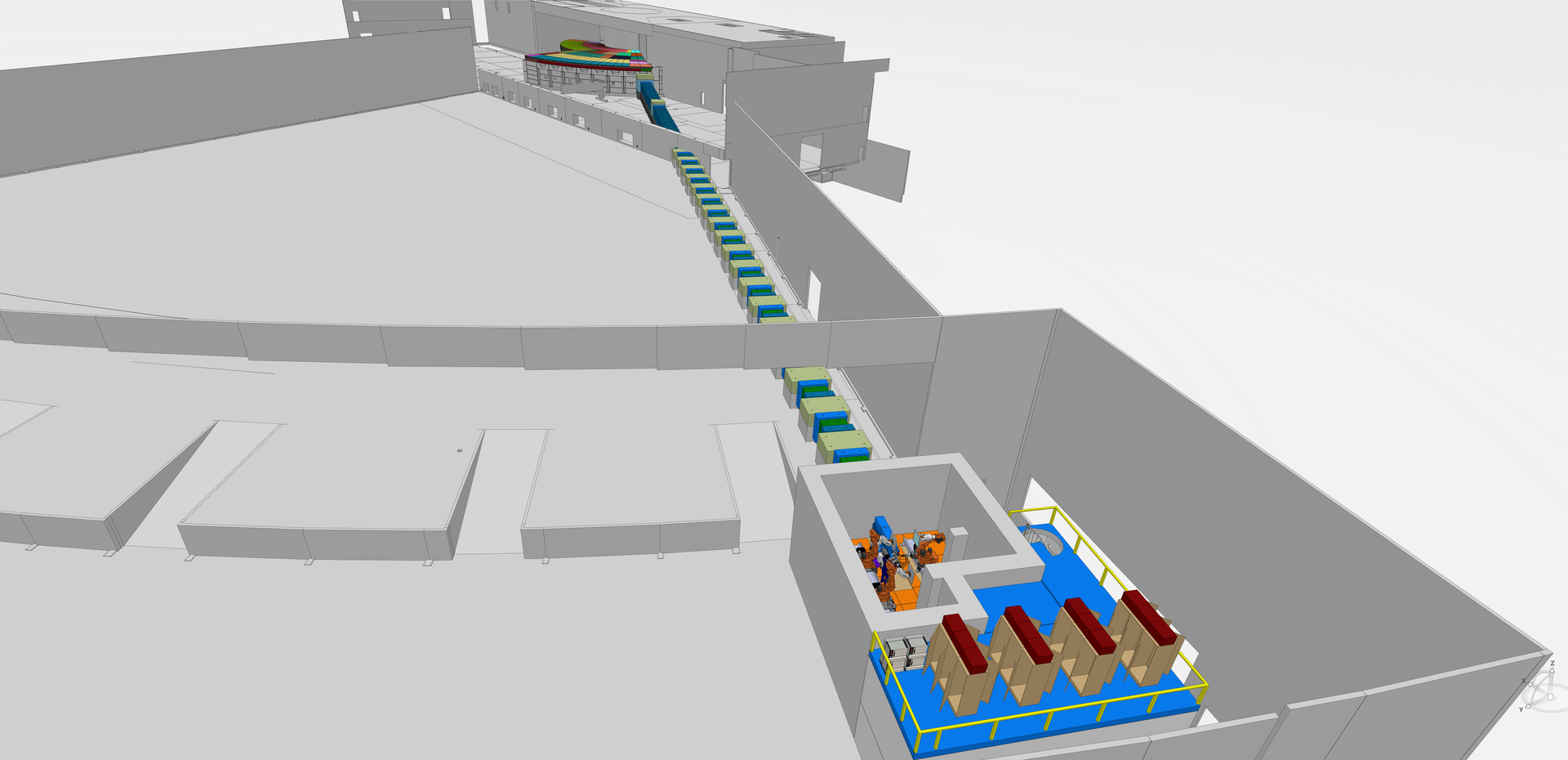
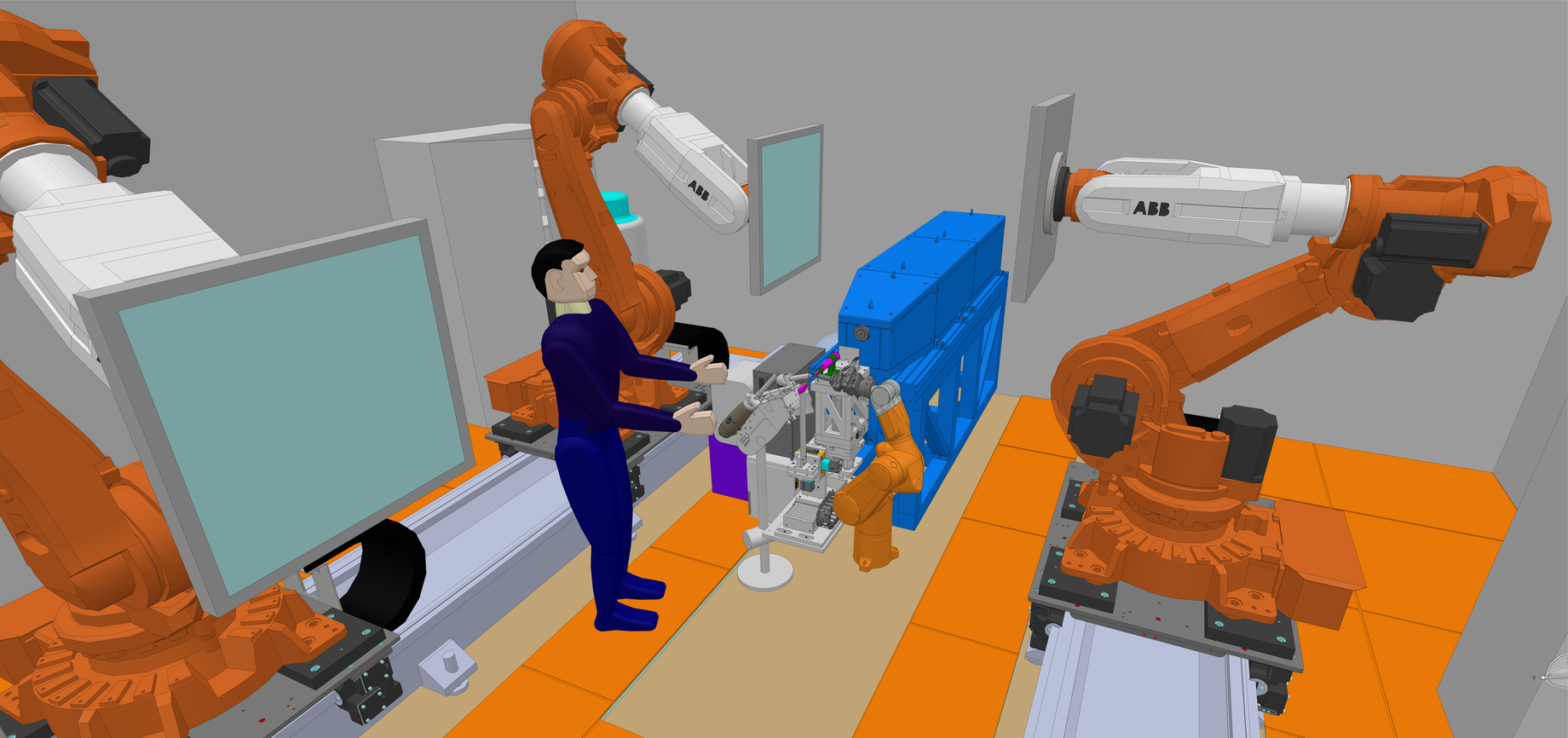
The design of NMX includes a trio of automated 6-axis robotic arms that mount and rotate the sample to the desired orientation, as well as position the neutron detectors. Individual robotic arms can also be upgraded with new functionalities, for example a tool changer, to automatically switch from one application to another. The robotics system is based on existing technology found in FIP-BM30A, the French beamline for Investigations on Protein Crystallography, and in the latest evolution of the G-ROB goniometer both at the European Synchrotron Radiation Facility (ESRF) in France.
In the early 2000s, conceptual development of the robotics at IBS and the European Molecular Biology Lab (EMBL) in France demonstrated that the user experience could be simplified by replacing functionalities for sample orientation and mounting normally found in separate elements of the sample setup such as a goniometer and magnetic head.
“There are two systems [in NMX] actually,” says Jean-Luc Ferrer, leader of the Synchrotron group (GSY) at IBS and NatX-ray company co-founder, who holds responsibility for the robotics design. “A detector handling system that our industrial partner NatX-ray is building for the ISIS facility right now, and a robotised goniometer, a shared development between IBS and NatX-ray.”
The robotic arms orient NMX’s three detectors, which record the neutron diffraction pattern scattered off a crystal sample. The flecked pattern produced, including the intensity of and distance between the spots, reveals information about the size and structure of a molecule.
“We need neutron detectors that can record these diffraction spots with extremely high resolution. We also need to detect them across a wide angular range around the sample,” explains Giuseppe Aprigliano, ESS lead instrument engineer for NMX. “The combination of these two needs is what requires the high flexibility, in positioning the detectors, that the robotic system can offer.” Aprigliano previously worked on construction of the P12, P13, and P14 EMBL X-ray beamlines for the PETRA III accelerator at the Deutsches Elektronen-Synchrotron (DESY) in Germany.
The NMX instrument utilises Gaseous Electron Multiplier (GEM) detectors with gadolinium as the neutron converter—a choice considered more efficient than boron depending on the sample type measured. The unique adjustability of the detector geometry provides an active coverage area of 1.08 m2 with a 0.2 mm effective pixel size, totalling 27 million pixels.
“The neutron detectors of NMX will be beyond the present state-of-the-art,” says Dorothea Pfeiffer, ESS detector scientist for the detector group. “Further, the technological development of the detectors is an excellent example of interdisciplinary collaboration. We are collaborating with CERN, the worldwide experts in gaseous detectors, and have had excellent progress, including a well-formulated proof-of-concept that performs according to specified requirements. We are now working to complete a full-scale prototype for the summer of 2018.”
Glimpse the future
NMX’s versatility for sample handling and optimal use of the ESS long neutron pulse underlies its firm potential for drug discovery of new pharmaceuticals and improved industrial enzymes. Pinpointing the location of hydrogen atoms in biological cells will reveal to us the most basic and essential mechanics in living systems.
“Something that would really move to neutrons now would be membrane proteins, for example transporters that drive, or are themselves driven by a flux of protons across a membrane,” says Nissen. “And actually, all our bioenergetics are in one way or another linked to such proton these transporting events—photosynthesis, oxidative phosphorylation, for example the way we derive energy from food in the mitochondria and so on.”
Above Oksanen’s desk at ESS hangs a letter from Hartmut Michel, a Nobel Prize winner in chemistry for his breakthrough work on membrane proteins with X-ray crystallography. In the letter, Michel stresses the great importance of neutrons for future crystallography research and the need to build NMX at ESS.
New discoveries—whether from X-ray or neutron research—and their societal impact are difficult to predict, explains Oksanen. “Having a broad and fundamental understanding of things is what produces the real innovations. If you’re working on something you know is going to be practically relevant then somebody else is probably working on that already. It’s known that the problem is there. But, when you look at something that is just interesting for you and other scientists then that’s where the really unexpected things come from.”