Highlighting the value of crystallography initiatives at ESS and the breakthrough promise of NMX, a longstanding collaboration between researchers at SNS, ESS, ILL and other labs is using neutrons to lay the foundation for better cancer drug therapies.
LUND—A recent study led by scientists at Oak Ridge National Laboratory (ORNL) in the US, collecting data on neutron scattering instruments at ORNL’s Spallation Neutron Source (SNS) and Grenoble’s Institut Laue-Langevin (ILL), has established a foundation for further study of drugs targeting aggressive cancers so precisely that side effects may be significantly mitigated. The key to this research was the identification of hydrogen positions within enzymes through neutron macromolecular crystallography. And the key to enabling the experiments was the development of crystal samples for the three glaucoma drugs used in the study.
DEMAX: Better Samples
European Spallation Source (ESS) scientist Zoë Fisher, who is responsible for developing the Deuteration and Macromolecular Crystallisation (DEMAX) laboratory platform for ESS, has been involved in research contributing to this work since 2012 and is a co-author of the recent paper.
“This work is super-exciting and part of a larger collaboration and long-term project spanning many research groups and large-scale facilities,” says Fisher. “We have been studying the carbonic anhydrase enzyme structure (CA) with neutrons, and now also with inhibitors bound, for a number of years. In 2012 we published the first neutron structure with a clinically-used drug bound in the active site of CA. Since then there have been many new developments in inhibitor development. This latest study adds a great deal to our knowledge base of inhibitor and enzyme interactions. Ultimately our goal is to use neutrons as a tool to better understand drug binding and then to use this information to make improved compounds with reduced side effects.”
NMX: Higher Flux
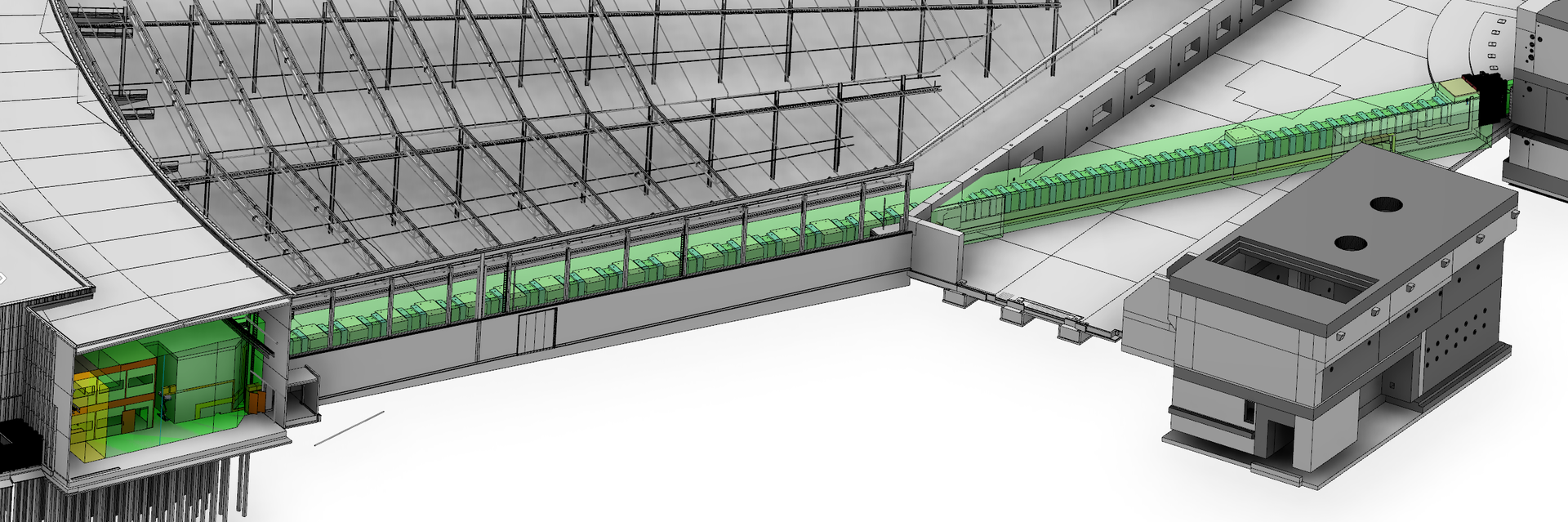
The high neutron flux generated by ESS will be a game-changer in the identification and characterisation of hydrogen atoms in complex molecular structures, like proteins and enzymes. NMX, the macromolecular crystallography instrument under construction for ESS, will be the world-leading instrument for these types of studies and will break new ground in structural biology. Allowing for smaller crystal samples and shorter measurement times, NMX will enable researchers to identify the position of hydrogen atoms in molecules where this basic information remains a mystery.
DEUNET: Stronger Network
The development of new deuteration and crystallisation methods at ESS is not only necessary to optimise the use of the new facility for life science studies, but will serve as a coordination point for the development of these processes worldwide. An important step in this direction was the inauguration of DEUNET last year, an initiative driven by ESS instrument scientist Hanna Wacklin-Knecht and supported by the EU’s SINE2020 grant project.
The story from ORNL’s website concerning the targeting of enzymes with glaucoma drugs is excerpted below. The full article is available on ORNL’s website.
Neutron Study of Glaucoma Drugs Offers Clues About Enzyme Targets for Aggressive Cancers
Oak Ridge National Laboratory
FEB 12, 2018
New insights from neutron analysis of glaucoma drugs and their enzyme target may help scientists design drugs that more effectively target aggressive cancers.
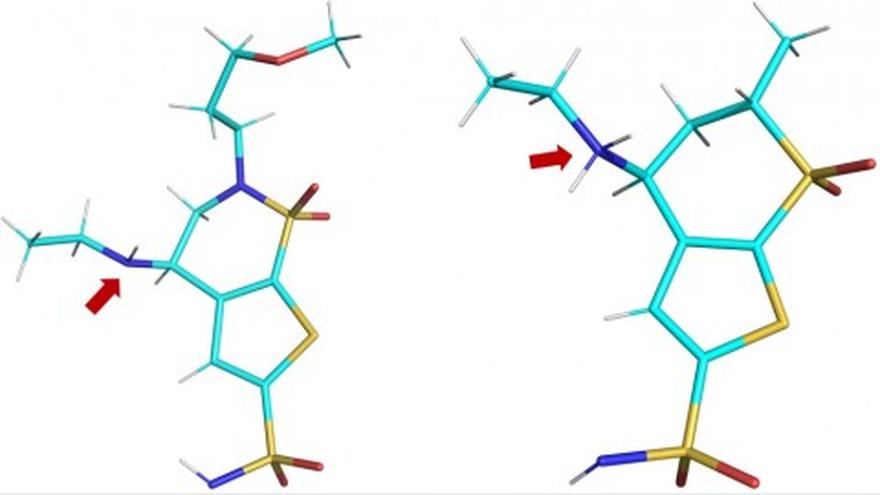
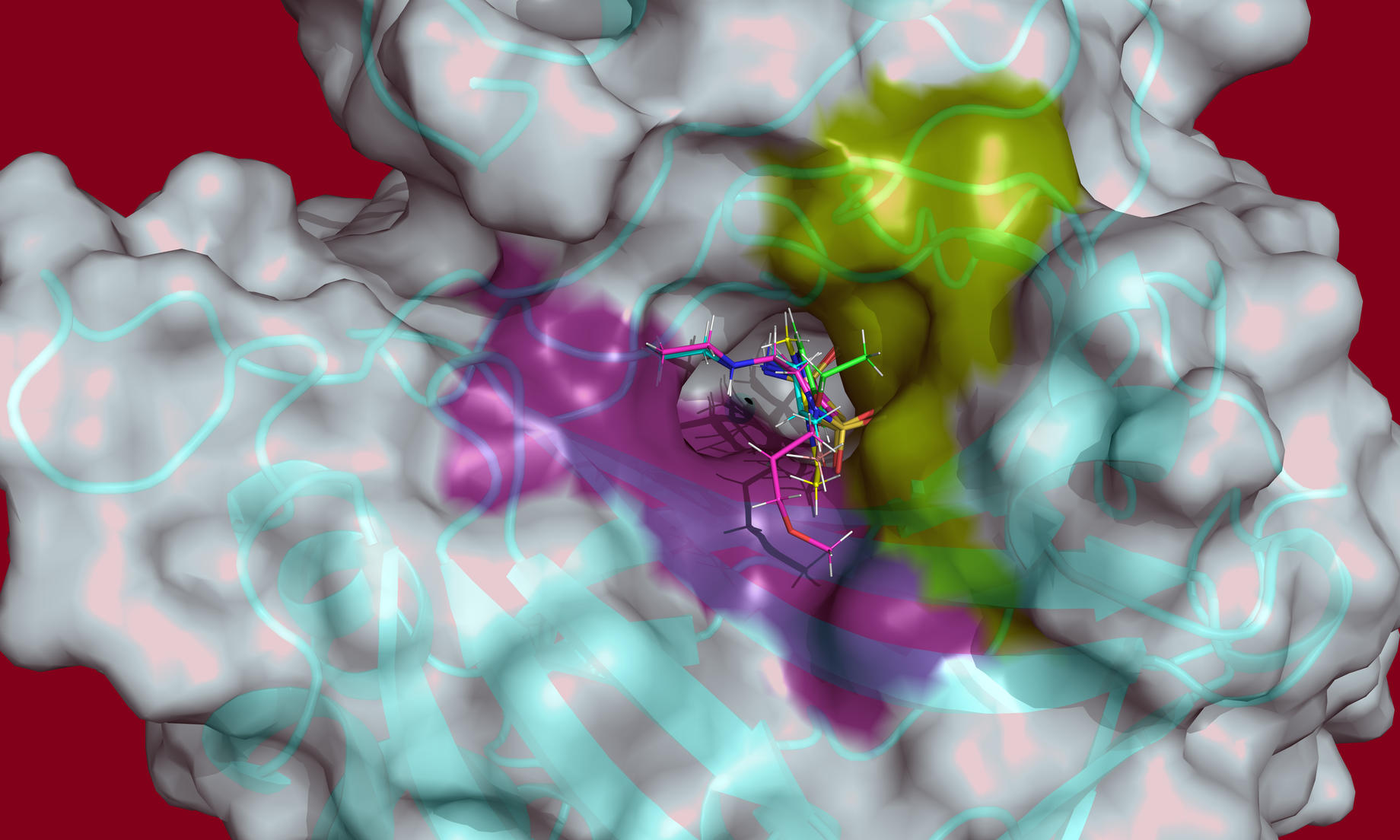
A team of researchers led by the Department of Energy’s Oak Ridge National Laboratory used neutron macromolecular crystallography to investigate the different states of three glaucoma drugs as they interact with the targeted enzyme, human carbonic anhydrase II (hCA II). The study, published in the journal Structure, found that temperature, pH, and the electrical charge of the three glaucoma drugs affected their ability to target and bind with the hCA II enzyme.
- “Our goal was to observe differences in the presentation of three clinically used glaucoma drugs while they are bound to the hCA II enzyme,” said Andrey Kovalevsky, an instrument scientist at ORNL and a senior co-author of the study. “By looking at how well these drugs target hCA II in protonated, neutral and deprotonated states, we hoped to obtain insights that would make it possible to improve these medicines so they can better target enzymes linked to cancer.”
- “This discovery was really a proof of principle for us,” said Robert McKenna, a professor at the University of Florida and a senior co-author of the study. “It opened our eyes to how changes in temperature and pH can impact the protonation state of the drug, which in turn makes it more or less effective.”
- “We want to exploit the difference in charge, pH and temperature to see if we can design drugs that are more effective at targeting these enzymes,” said Kovalevsky. “If we can understand binding at the atomic level, we can redesign drugs and turn them into stronger and more selective ‘magnets’ that will be attracted to cancer-associated enzymes. Such drugs would be much more effective at killing cancer cells while leaving healthy cells unhurt, which significantly reduces side effects for patients.”
- “When you use neutron diffraction you don’t have radiation damage, so you can do your study at room temperature,” said McKenna. “In addition, freezing crystals may alter the drug and enzyme, introducing a false view into the study, while room temperature studies more closely resemble the environment the drug will be used in.”
Kovalevsky et al., "To Be or Not to Be" Protonated: Atomic Details of Human Carbonic Anhydrase-Clinical Drug Complexes by Neutron Crystallography and Simulation, Structure (2018), https://doi.org/10.1016/j.str.2018.01.006