Neutron reflectometry has given scientists an atomic-level insight into the behaviour of Bcl-2, a protein that can contribute to cancerous cell growth. The study was carried out by scientists from Sweden's Umeå University in collaboration with the research facilities ISIS (UK) and ESS, and the first paper has been published in the journal Nature Communications Biology.
Elevated function of the cell-protecting membrane protein Bcl-2 can promote cancer and cause resistance to cancer treatment. Developing an understanding of the way it does this could inform the development of anti-cancer drugs.
It may seem counter-intuitive, but cell death is crucial to overall health, and is managed by a series of proteins from the Bcl-2 family. These proteins work together at the membrane surface of intracellular organelles – the mitochondria, to determine a cell’s wellbeing. For all that, over-production of the cell-protecting Bcl-2 members can interrupt this delicate balance and inhibit signals for cell death. This can cause cancerous cells to continue to grow, and not respond to cancer treatment.
However, how cell-protecting and cell-killing proteins of the Bcl-2 family interact with one another in their intracellular membrane environment is not fully understood, since a picture of their structure and behaviour in this environment was not available.
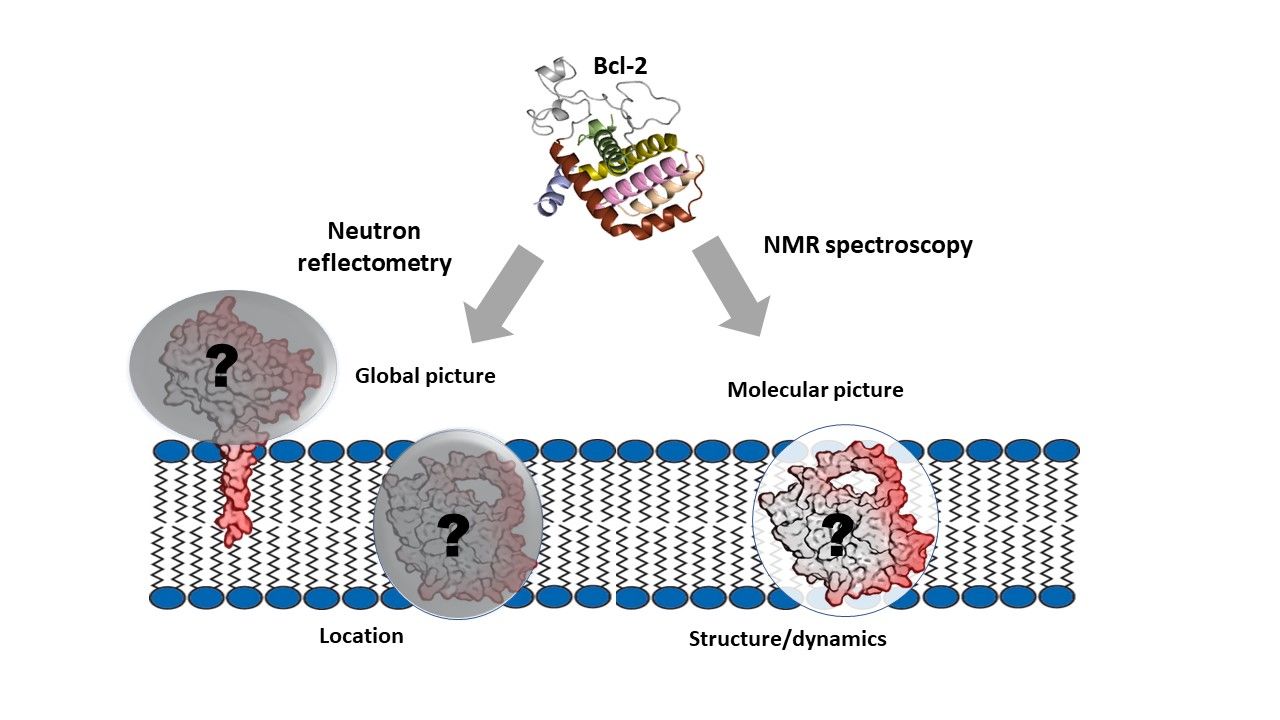
In this study, published in Nature Communications Biology, the researchers used the novel combination of neutron reflectometry and NMR spectroscopy to study full length human Bcl-2 protein located in a membrane environment, providing insight into the key structural and dynamic features.
The collaboration is fruit of the Swedish Research Council’s (Vetenskapsrådet) project grants awarded in 2016, that aimed to promote neutron research in Sweden by enabling scientists to learn using neutron scattering for their research, in order to become early users at ESS, once operations start at the facility in Lund.
Collaboration is key
ESS Life Scientist, Dr Hanna Wacklin-Knecht, contributed in this research collaboration with expertise to optimise samples and experiment conditions for neutron reflectometry, and by helping the Umeå chemists to do preliminary characterisation at Lund University’s Physical chemistry Division, where she is Adjunct Senior Lecturer.
“The research project with Professor Gröbner is an excellent example of how close collaboration with the research facilities ESS and ISIS helps new research groups use neutrons in their pioneering research and prepares them to become users of ESS," says Hanna Wacklin-Knecht, ESS Life Scientist.
The NR experiments were performed in collaboration with Dr Luke Clifton at the ISIS neutron source. These investigations enabled the researchers to determine the relative distribution of Bcl-2 protein across the membrane. The results showed that the protein sits within the membrane rather than on the surface, as previously thought.
Their NMR experiments looked at individual protein segments and their behaviour in the membrane, and suggest that the part of the protein that acts as a molecular switch is located on, or close to, the membrane interface. However, the main protein body that blocks the cell-killing partners, is restricted within the membrane.
Scientific breakthrough
The experiments have led to a significant breakthrough in the understanding of how Bcl-2 exerts its cell-protecting function at the membrane level by simply inhibiting cell-killing family members there.
“By integrating neutron reflectometry (NR) and NMR spectroscopy we were able to uncover the location and behaviour of the Bcl-2 protein in its native membrane,” explains study author Gerhard Gröbner; “It is a breakthrough, not only in understanding the molecular cell-protecting function of Bcl-2, but also its notorious role in cancers, thereby making this protein a prime target in the hunt for novel cancer therapies.”
Since this first study that has been published in Nature Communications Biology, the researchers in the collaboration have continued their research with many additional experiments – some remotely during the pandemic, as ISIS has remained open.
The ESS DEMAX laboratory provided valuable services for this work
When the ESS Deuteration & Macromolecular Crystallisation platform (DEMAX) opened in 2019, Gerhard Gröbner was one of the first researchers to make use of the services of this laboratory. As a result, Hanna Wacklin-Knecht has, through the DEMAX pilot proposal calls, provided deuterated lipids for the follow-up studies that have been conducted on the function of Bcl-2s. These collaborative studies also help developing the capabilities of DEMAX, by building knowledge that will benefit future research using neutrons.
“Neutrons give an advantage because of the deuterium labelling – but each time we have to learn how to best produce the molecules needed for the particular experiment,“ explains Hanna Wacklin-Knecht. “Our work at DEMAX for Gerhard Gröbner will be useful for other researchers and other facilities – because the lipid molecules are also relevant in other experiments by other users.”
In future experimental studies on the Bcl-2s, the researchers hope to discover how the position in the membrane is related to the way that it prompts cell death.
“Together, we now plan to unravel the active state of Bcl-2 protein when caught in the act of binding cell-killing proteins at the membrane,” concludes Gerhard Gröbner.
A. U. Mushtaq, J. Ådén , L. A. Clifton , H. Wacklin-Knecht , M. Campana , A. P. G. Dingeldein, C. Persson, T. Sparrman, and G. Gröbner: Neutron reflectometry and NMR spectroscopy of full length Bcl-2 protein reveal its membrane localization and conformation. Nature Communications Biology (2021). Doi: 10.1038/s42003-021-02032-1